کیت استخراج پلازمید با استفاده از ستون | Plasmid DNA Isolation kit
14,700,000 ریال
توضیحات محصول
کیت استخراج پلازمید با استفاده از ستون | Plasmid DNA Isolation kit
KIASMID
Plasmid DNA Isolation Kit
Cat. No:
FPKT019.0025
FPKT019.0050
FPKT019.00100
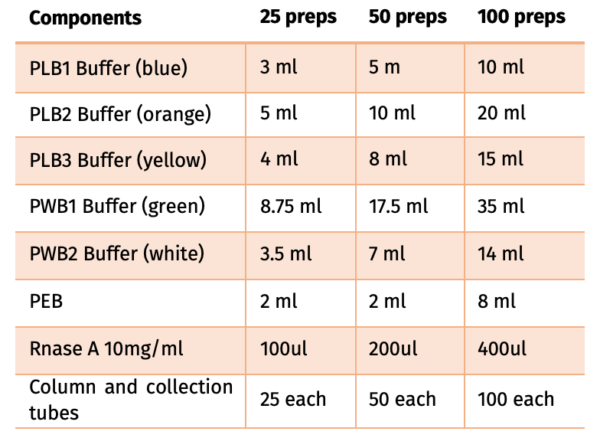
Contents:
Kit storage:
![]() This kit should be stored at room temperature.
This kit should be stored at room temperature.
![]() RNase A should be stored at -20 °C
RNase A should be stored at -20 °C
![]() If properly stored, all kit components are stable until the expiration date printed on the label.
If properly stored, all kit components are stable until the expiration date printed on the label.
![]() After adding RNase, A to PLB1 buffer and absolute ethanol to wash buffers, Store the PLB1 and wash Buffers at +2 to +8°C
After adding RNase, A to PLB1 buffer and absolute ethanol to wash buffers, Store the PLB1 and wash Buffers at +2 to +8°C
Additional Equipment and Reagent required
- Isopropyl alcohol
- Standard tabletop microcentrifuge capable of 13,000 x g centrifugal force
- Microcentrifuge tubes, 1.5 ml, sterile
- Absolute ethanol
Application:
Isolation of up to 15μg purified plasmid DNA from bacterial cultures, which may be used directly in downstream applications such as restriction enzyme digestion, PCR, cloning, sequencing, in vitro transcription, or labeling reactions. The procedure is optimized to achieve reliable results within 90 min.
Handling Requirements and Safety Information
![]() All solutions are clear and should not be used when precipitates have formed. Warm the solutions at +15 to +25°C or in a 37°C water bath until the precipitates have dissolved.
All solutions are clear and should not be used when precipitates have formed. Warm the solutions at +15 to +25°C or in a 37°C water bath until the precipitates have dissolved.
![]() PLB3 and PWB1 Buffers contain guanidinium which is an irritant.
PLB3 and PWB1 Buffers contain guanidinium which is an irritant.
![]() Do not allow PLB3 and PWB1 Buffers to touch your skin, eyes, or mucous membranes. If contact does occur, wash the affected area immediately with large amounts of water.
Do not allow PLB3 and PWB1 Buffers to touch your skin, eyes, or mucous membranes. If contact does occur, wash the affected area immediately with large amounts of water.
![]() If you spill the reagent, dilute the spill with water before wiping it up.
If you spill the reagent, dilute the spill with water before wiping it up.
![]() Do not use any modified ethanol.
Do not use any modified ethanol.
![]() Do not pool reagents from different lot numbers.
Do not pool reagents from different lot numbers.
![]() Immediately after usage, close all bottles in order to avoid leakage, varying buffer concentrations or buffer conditions.
Immediately after usage, close all bottles in order to avoid leakage, varying buffer concentrations or buffer conditions.
![]() After first opening store all bottles in an upright position.
After first opening store all bottles in an upright position.
![]() Do not allow the PLB3 and PWB1 Buffers to mix with sodium hypochlorite found in commercial bleach solutions. This mixture can produce a highly toxic gas.
Do not allow the PLB3 and PWB1 Buffers to mix with sodium hypochlorite found in commercial bleach solutions. This mixture can produce a highly toxic gas.
![]() Wear protective disposable gloves, laboratory coats and eye protection, when handling samples and kit reagents.
Wear protective disposable gloves, laboratory coats and eye protection, when handling samples and kit reagents.
![]() Do not contaminate the reagents with bacteria, virus, or nucleases. Use disposable pipets and nuclease free pipet tips only, to remove aliquots from reagent bottles.
Do not contaminate the reagents with bacteria, virus, or nucleases. Use disposable pipets and nuclease free pipet tips only, to remove aliquots from reagent bottles.
preparation procedure:
Working Solution Preparation
![]() add RNase A to PLB1 Buffer completely Dissolve the RNase A by vortexing. store the PLB1 buffer at 4 °C.
add RNase A to PLB1 Buffer completely Dissolve the RNase A by vortexing. store the PLB1 buffer at 4 °C.
FPKT019.0025-25 preps
![]() Add 3.75 ml Isopropyl alcohol to PWB1 Buffer Bottle.
Add 3.75 ml Isopropyl alcohol to PWB1 Buffer Bottle.
![]() Add 14 ml absolute ethanol to PWB2 Buffer Bottle.
Add 14 ml absolute ethanol to PWB2 Buffer Bottle.
FPKT019.0050-50 preps
![]() Add 7.5 ml Isopropyl alcohol to PWB1 Buffer Bottle.
Add 7.5 ml Isopropyl alcohol to PWB1 Buffer Bottle.
![]() Add 28 ml absolute ethanol to PWB2 Buffer Bottle.
Add 28 ml absolute ethanol to PWB2 Buffer Bottle.
FPKT019.0100-100 preps
![]() Add 15 ml Isopropyl alcohol to PWB1 Buffer Bottle.
Add 15 ml Isopropyl alcohol to PWB1 Buffer Bottle.
![]() Add 56 ml absolute ethanol to PWB2 Buffer Bottle.
Add 56 ml absolute ethanol to PWB2 Buffer Bottle.
![]() Please note that the Ethanol concentration of a Washing Buffer may decrease during long term storage resulting in a drop-down of the final DNA yield.
Please note that the Ethanol concentration of a Washing Buffer may decrease during long term storage resulting in a drop-down of the final DNA yield.
![]() before starting Incubate the PEB buffer at 55 to 65 ° C until the end of the protocol to obtain the maximum yields.
before starting Incubate the PEB buffer at 55 to 65 ° C until the end of the protocol to obtain the maximum yields.
Protocol
- Sample preparation
Pellet the bacterial cells from 1-5 ml of E. coli culture (OD600= 1.5-5 per ml) by centrifuge in 8000g for 1 min. Discard the supernatant (Do not use more highly concentrated sample)
- Add 100 μl chilled PLB1 Buffer (with RNase A) to the bacterial pellet. Resuspend the bacterial pellet by vortex for 30 s.
- Add 200 μl PLB2 Buffer to the mixture. Mix gently by inverting the tube 5 to 10 times (Do not vortex). Incubated at room temperature for 1 min (Do not incubate more).
- Add 150 μl chilled PLB3 Buffer. Mix gently by inverting the tube 5 to 10 times (Do not vortex). Incubated on ice for 5 min (The solution should become cloudy and a flocculent precipitate should form).
- Centrifuge at Maximum speed for 5 min at +4°C and transfer entire supernatant into DNA spin column.
- Centrifuge at Maximum speed for 1 min at room temperature. Disconnect the Filter Tube, and discard the flow through solution. Reconnect the Filter Tube to the same Collection Tube.
- (Optional: this step Removes nucleases – if you need higher plasmid extraction yield go to next step and don’t add PWB1 buffer) Add 500 μl PWB1 buffer to the center of column. Centrifuge at Maximum speed for 1 min at room temperature and discard the flow through. (better to do for strains which have high nuclease content such as HB101 or JM strains. This step may reduce the final concentration of extracted plasmid although you will obtain more pure plasmid.)
- Add 700 μl PWB2 to DNA spin column.
- Centrifuge 1 min at maximum speed. Discard the flow through solution.
- Centrifuge the DNA spin column at Maximum speed for 1 min to remove residual ethanol. Reconnect the Filter Tube to a clean 1.5 ml microcentrifuge tube.
- Add 50 μl PEB Buffer or sterile DDW directly onto column membrane and stand for 3 min.
- Centrifuge at Maximum speed for 1 min.
- Check 5-10 μl of extracted plasmid DNA by 1% agarose gel electrophoresis. Store Plasmid DNA at -20˚C
بررسی تخصصی
| تعداد Preparation را انتخاب کنید | 100, 50, 25 |
|---|


















علی –
قیمت و کیفیت عالی
inhiple –
All of CI values listed in Figure 3B were
inhiple –
The problem with Clomid is that, even while it promotes conception, its continued presence in the mother s body threatens the health of the resulting embryo