کیت استخراج RNA از سلول و بافت|Cell and Tissue RNA isolation kit
16,000,000 ریال
توضیحات محصول
کیت استخراج RNA از سلول و بافت|Cell and Tissue RNA isolation kit
KIARNA
High Pure Cell and Tissue RNA extraction kit
Cat. No:
FPKT030.0025
FPKT030.0050
FPKT030.0100
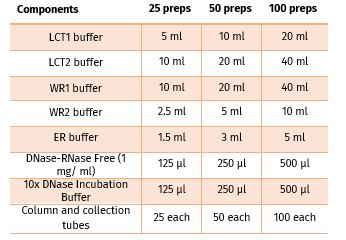
Contents:
Kit storage:
![]() This kit should be stored at room temperature.
This kit should be stored at room temperature.
![]() DNase should be stored at -20 °C
DNase should be stored at -20 °C
![]() If properly stored, all kit components are stable until the expiration date printed on the label.
If properly stored, all kit components are stable until the expiration date printed on the label.
Additional Equipment and Reagent required
- Make sure everything is RNase-free when handling RNA.
- Absolute ethanol
- ß-Mercaptoethanol
- Standard tabletop microcentrifuge capable of 13,000 x g centrifugal force
- Microcentrifuge tubes, 1.5 ml, sterile
- liquid nitrogen & mortar
- a rotor-stator homogenizer or a 20-G needle syringe
Application
The High Pure RNA Isolation Kit is designed for the purification of total RNA from Tissue and cultured cell.
Due to the integrated DNase digestion step, contamination of the isolated RNA with residual genomic DNA is mostly avoided. In addition, RNA is suited for other techniques like northern blotting, RNase protection and primer extension. The procedure is optimized to achieve reliable results within Approximately 1 hour (24 samples simultaneously).
Handling Requirements and Safety Information
![]() All solutions are clear and should not be used when precipitates have formed. Warm the solutions at +15 to +25°C or in a 37°C water bath until the precipitates have dissolved.
All solutions are clear and should not be used when precipitates have formed. Warm the solutions at +15 to +25°C or in a 37°C water bath until the precipitates have dissolved.
![]() LCT2 Buffer and WR1 Buffer contain guanidinium hydrochloride which is an irritant.
LCT2 Buffer and WR1 Buffer contain guanidinium hydrochloride which is an irritant.
![]() ß-Mercaptoethanol (ß-Me) is hazardous to human health. perform the procedures involving ß-Me in a chemical fume hood.
ß-Mercaptoethanol (ß-Me) is hazardous to human health. perform the procedures involving ß-Me in a chemical fume hood.
![]() Do not allow LCT2 Buffer and WR1 Buffer to touch your skin, eyes, or mucous membranes. If contact does occur, wash the affected area immediately with large amounts of water.
Do not allow LCT2 Buffer and WR1 Buffer to touch your skin, eyes, or mucous membranes. If contact does occur, wash the affected area immediately with large amounts of water.
![]() If you spill the reagent, dilute the spill with water before wiping it up.
If you spill the reagent, dilute the spill with water before wiping it up.
![]() Do not use any modified ethanol.
Do not use any modified ethanol.
![]() Do not pool reagents from different lot numbers.
Do not pool reagents from different lot numbers.
![]() Immediately after usage, close all bottles in order to avoid leakage, varying buffer concentrations or buffer conditions.
Immediately after usage, close all bottles in order to avoid leakage, varying buffer concentrations or buffer conditions.
![]() After first opening store all bottles in an upright position.
After first opening store all bottles in an upright position.
![]() Do not allow the LCT2 Buffer and WR1 Buffer to mix with sodium hypochlorite found in commercial bleach solutions. This mixture can produce a highly toxic gas.
Do not allow the LCT2 Buffer and WR1 Buffer to mix with sodium hypochlorite found in commercial bleach solutions. This mixture can produce a highly toxic gas.
![]() Wear protective disposable gloves, laboratory coats and eye protection, when handling samples and kit reagents.
Wear protective disposable gloves, laboratory coats and eye protection, when handling samples and kit reagents.
![]() Do not contaminate the reagents with bacteria, virus, or nucleases. Use disposable pipets and nuclease free pipet tips only, to remove aliquots from reagent bottles.
Do not contaminate the reagents with bacteria, virus, or nucleases. Use disposable pipets and nuclease free pipet tips only, to remove aliquots from reagent bottles.
preparation procedure:
Working Solution Preparation
FPKT030.0025-25 prep
![]() Add 2.5 ml absolute ethanol to WR1 Bottle.
Add 2.5 ml absolute ethanol to WR1 Bottle.
![]() Add 10 ml absolute ethanol to WR2 Bottle.
Add 10 ml absolute ethanol to WR2 Bottle.
FPKT030.0050-50 prep
![]() Add 5 ml absolute ethanol to WR1 Bottle
Add 5 ml absolute ethanol to WR1 Bottle
![]() Add 20 ml absolute ethanol to WR2 Bottle
Add 20 ml absolute ethanol to WR2 Bottle
FPKT030.0100-100 prep
![]() Add 10 ml absolute ethanol to WR1 Bottle.
Add 10 ml absolute ethanol to WR1 Bottle.
![]() Add 40 ml absolute ethanol to WR2 Bottle.
Add 40 ml absolute ethanol to WR2 Bottle.
![]() Please note that the Ethanol concentration of a Washing Buffer may decrease during long term storage resulting in a drop-down of the final RNA yield.
Please note that the Ethanol concentration of a Washing Buffer may decrease during long term storage resulting in a drop-down of the final RNA yield.
![]() before starting Incubate the ER buffer at 55 to 65 ° C until the end of the protocol to obtain the maximum yields.
before starting Incubate the ER buffer at 55 to 65 ° C until the end of the protocol to obtain the maximum yields.
Protocols
- Collect 1 ~ 5 ×106 cells by centrifuge at 300 x g for 5 min at 4 °C. Remove all the supernatant. For Tissue Sample Weight up to 30 mg of tissue sample. Grind the sample in liquid nitrogen to a fine powder with a mortar and transfer the powder to a new microcentrifuge tube (not provided).
![]() Avoid thawing the sample during weighing and grinding.
Avoid thawing the sample during weighing and grinding.
- Resuspend cells in 200 μl LCT1 Buffer. Don’t add this Buffer to tissue Samples.
![]() Do not overload, too much sample will make cell lysis incompletely and lead to lower RNA yield and purity.
Do not overload, too much sample will make cell lysis incompletely and lead to lower RNA yield and purity.
- Add 400 μl LCT2 Buffer and 3.5 μl of ß-Mercaptoethanol and Vortex vigorously for 1 min to resuspend the cells completely. For Tissue Sample Homogenize the sample by using a rotor-stator homogenizer or by passing the sample lysate through a 20-G needle syringe 10 times. Incubate at room temperature for 5 min.
- Add 600 μl Absolute ethanol to the homogenized lysate, and mix well by pipetting.
![]() If the clump is still visible after vortex, pipet sample mixture up and down to break down the clump.
If the clump is still visible after vortex, pipet sample mixture up and down to break down the clump.
- To transfer the sample to a High Pure Filter Tube:
-Insert one High Filter Tube in one Collection Tube.
-Pipet entire sample into the upper reservoir of the Filter Tube (max. 800 μl). Insert the entire High Pure Filter Tube assembly into a standard tabletop centrifuge. Centrifuge the tube assembly 1 min at ≥8000 xg (≥10,000 rpm). - Optional Step: DNase I digestion to eliminate genomic DNA contamination follow the step from 6a. Otherwise proceed to step 8 directly.
6a. Discard the flow through liquid. Add 250 μl of WR1 to the Filter Tube, centrifuge 30 sec at ≥8000 xg (≥10,000 rpm).
6b. Discard the flow through liquid. For each sample, pipette 5 µl of 10x DNase incubation buffer into a new sterile microtube and add 5 µl of DNase I. with DEPC-treated water reach to a volume of 50 µl, then pipette the solution into the upper reservoir of the filter tube. Incubate for 15 min at +15 to +25°C.
6c. Discard the flow through liquid. Add 250 μl of WR1 to the Filter Tube, centrifuge 30 sec at ≥8000 xg (≥10,000 rpm).
- Discard the flow through liquid. Add 500 μl WR1 to the upper of the Filter Tube and centrifuge 30 sec at ≥8000 xg (≥10,000 rpm).
- Discard the flow through liquid. Add 500 μl WR2 to the upper of the Filter Tube. centrifuge 30 sec at ≥8000 xg (≥10,000 rpm). and discard the flow through.
- Leave the tube assembly in the centrifuge and spin it for 3 min at maximum speed (approximately 14,000 rpm) to remove any residual Wash Buffer.
- Discard the Collection Tube and insert the Filter Tube into a clean, sterile RNase free and DNase free 1.5 ml microcentrifuge tube.
- Add 50 μl ER Buffer to the upper of the Filter Tube. Centrifuge the tube assembly for 1 min at ≥8000 xg (≥10,000 rpm).
to obtain the maximum yields you can add the RNA solution to the top of the filter tube and repeat Centrifuge the tube assembly at ≥8000 xg (≥10,000 rpm).
The microcentrifuge tube now contains the eluted Cell RNA. Either use the eluted RNA directly in RT.PCR or store the eluted RNA at −20°C for later analysis.
بررسی تخصصی
| سایر ویژگیها | ||
|---|---|---|
|
||



















علی –
قیمت و کیفیت عالی
پرند –
ممنون، خوب بود